

In The Current State of Drug Price Transparency Reporting webinar that recently aired , Rupal Patel, Senior Director of Advisory Services, and John Whitridge, Manager of Advisory Services, discussed a growing concern with pharmaceutical manufacturers: State Pricing Transparency Reporting (SPTR). Here is a breakdown of what was discussed, how it complicates the regulatory landscape, and what manufacturers can do to mitigate risk.
Over the past few years, almost half of all states have signed, or are in the process of signing into law pharmaceutical pricing transparency measures which place new compliance and reporting obligations on manufacturers. These new regulations are designed to limit drug costs by requiring manufacturers to report on drug prices and price increases.
State Pricing Transparency legislation is a response to the industry trend of increasing Wholesale Acquisition Cost (WAC) prices and the lack of public knowledge around the reasoning for those increases. Individual states have taken it upon themselves to take legislative action in order to raise awareness for drug pricing trends over time allowing more accurate cost forecasts for state budgets.
State Pricing Transparency Reporting presents a set of challenges to manufacturers, such as:
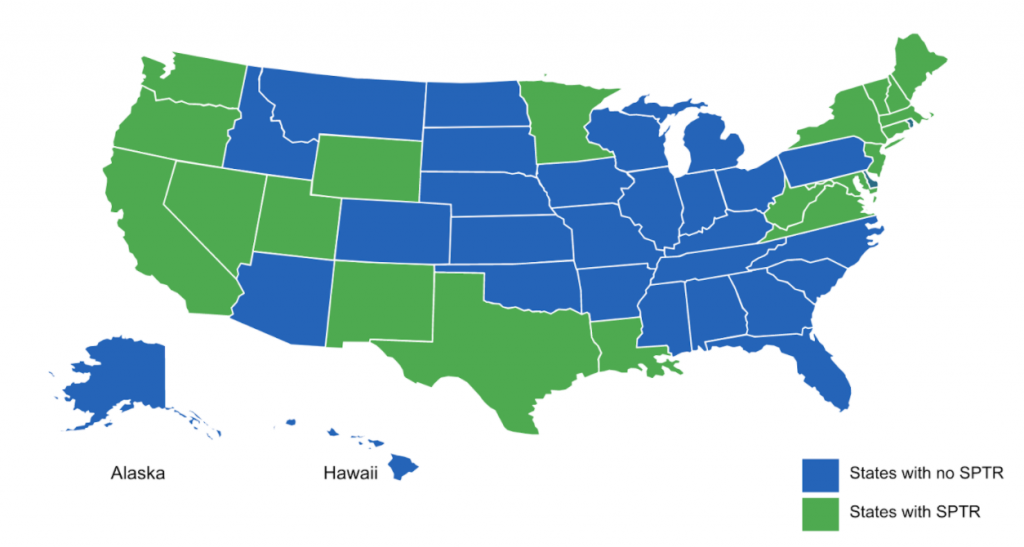
This is a snapshot of states that as of 2021, have enacted or expanded state price transparency laws in addition to current state legislation
It’s likely that this type of legislation will continue as more states seek to control the cost of pharmaceuticals in their annual budget. The Biden administration has indicated that they support the public knowledge of drug pricing and there is the potential for federal-level policies to complement state regulations.
When speaking with a current IntegriChain customer, an SVP of Market Access at a mid-market pharmaceutical manufacturer, he had this to say about his decision to use IntegriChain’s state pricing transparency reporting:
“We were getting a patchwork in regards to state price reporting requirements and other state obligations. There are 50 states, all with different reporting requirements and there’s only one of me. It’s a lot to keep up with since it’s a constantly evolving area. Since it’s so much to manage, I believe it is frequently overlooked within the market access world, but it can really hurt you if you’re not paying attention to it.”
The first thing to know is that you don’t have to go it alone. IntegriChain is a trusted partner to over 60 pharmaceutical manufacturers, and can help with:
In addition, IntegriChain can also communicate with state agencies. Since many bills have vague language, being able to confirm unique contracting or manufacturing scenarios directly with state agencies can save your organization time and trouble.
And most importantly, our subject matter experts know that constant monitoring and awareness is key , since:
If you’re interested in achieving operational readiness and reducing compliance risk, reach out to price.transparency@integrichain.com.
Click here to hear a full replay of this webinar.

Executive Director, Advisory Services
Rupal Patel is Executive Director in IntegriChain’s Operational Consulting practice, responsible for overseeing and leading the Government Pricing Advisory team. She is a recognized trusted advisor to Life Sciences manufacturers with an extensive record of success delivering strategic solutions that improve organizational accuracy, efficiency, and compliance. Currently she oversees more than 150 small- to mid-sized manufacturers and has extensive experience in leading pre-commercial launch projects for both Government Pricing and State Price Transparency.

Director, Operational Consulting
John Whitridge has spent many years working with manufacturers in a consulting role before shifting his attention to growing his expertise in State Price Transparency Reporting at IntegriChain. His background includes successful project execution in many areas such as system implementation, managed care contracting, chargeback validation, and government pricing. He is currently overseeing a dedicated team of SPTR resources. John looks to expand the offering and opportunities to support manufacturers in the complex waters of compliance.

Contracts & Pricing 



Contracts & Pricing
September 4, 2024Building pharma’s data-driven commercialization platform – from strategy to operational excellence.
8 Penn Center
1628 JFK Boulevard
Philadelphia, PA 19103
609.806.5005